
Intravenous Device research and practice
A new campaign aimed at raising awareness about the important and abundant use of intravenous (IV) devices in modern healthcare, the rate of potentially preventable complications, and the practice and products we can use to prevent these has been launched by a group of multi-disciplinary clinicians and researchers here in Australia.
Improved IV insertion and practice could spare patients a lot of unnecessary distress and discomfort, and save Australia’s health budget millions of dollars, says QUT School of Nursing Professor Samantha Keogh.
“Around 90 per cent of hospital patients in Australia require an IV to be inserted into their blood vessels to deliver vital drugs or fluids, or for monitoring and blood tests.
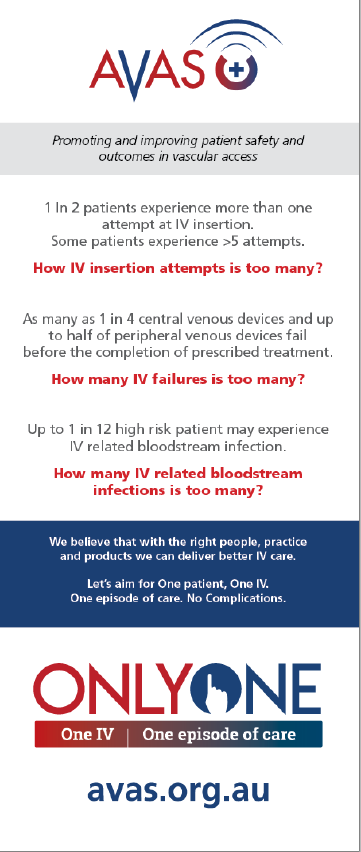
“Unfortunately, 1 in 2 patients experience more than one attempt at (peripheral) IV insertion. With some patients experiencing more than 5 attempts! Following insertion, as many as 1 in 4 central IV devices and up to half of peripheral IV devices fail before the completion of therapy (due to blockage, dislodgment or local inflammation). And up to 1 in 12 high risk patients may experience a serious IV related blood stream infection.”
Failed IVs need to be removed and replaced, which means repeated painful needlesticks for the patient and interruptions to vital treatment.
“Such failure rates for an essential device are unacceptable, especially as most failure is preventable with good insertion and maintenance practice,” said Professor Keogh, who was previously the president of the Australian Vascular Access Society (AVAS).
“AVAS’s ‘Only One’ campaign is based on ‘One patient. One IV. One episode of care. No complications.
“The goal is to promote and implement the latest evidence into clinical practice in Australia so that a patient needs just one jab to insert the needle correctly, that the catheter doesn’t block or fall out, introduce infection and therefore treatment is completed without complication.”
“AVAS’s campaign draws on the principles of the successful ‘bundle’ for central IV insertion developed by the US’s John Hopkins Medical Centre, where a concerted shift in culture and practice resulted in dramatically reduced IV-related infection rates.
“If we reduced failed (peripheral) IV insertions and failure by just 10 per cent, Australia could save over $175 million and prevent more than 2.5 million unnecessary needle sticks for patients.”
Approximately 30 million IV devices are used in Australia every year and this number is expected to rise to 46 million by 2025.
“The Only One campaign promotes (amongst other things) the use of dedicated and ultrasound trained IV inserters to improve the first insertion rate (of peripheral IVs) from 50 per cent to 95 percent., and risk of reduce adverse events with insertion of central IVs.
“While measures to reduce IV-related bloodstream infections have been highly successful, patients with ‘central’ IV catheters (inserted into a vein in the neck, chest or groin to deliver chemotherapy, antibiotics, and nutrients), approximately 10,000 (high risk) patients still experience IV-related bloodstream infections in Australian hospitals each year. Each infection costs the healthcare system (on average) $50,000 to manage in addition to discomfort, distress and extended hospital time for the patient.
Professor Keogh and fellow QUT Professor Ray Chan are co-researchers on an NHMRC-funded project led by AVATAR Group Director Professor Claire Rickard (based at University of Queensland) to test new products aimed at reducing complications associated peripherally inserted central catheters (PICCs) inserted into the central circulation via a vein in the arm and used to deliver specific forms of chemotherapy, drugs, fluids, and nutrition. (I would have a visual for this, indeed, all devices to demonstrate use and difference. We have plenty of slides.)
“Around 30% of PICCs fail from vascular, infectious or mechanical complications. Patients with cancer are at highest risk, and this increases morbidity, mortality and costs. Effective PICC dressing and securement may prevent PICC complications and failure” say Professor Rickard
“New products are available that may reduce these complications and this trial will test their effectiveness, costs, comfort and practicality for patients, nurses and doctors.”
The AVATAR Group was established to conduct rigorous and independent scientific trials of IV products and practices, implement best practice, and improve patient and healthcare outcomes.
“Our goal is to make vascular access complications history.”
* Note: In August 2012 Australian Health Ministers agreed to the first set of Australian Safety and Quality Goals for Health Care (the Goals). These Goals are:
- Safety of care: That people receive health care without experiencing preventable harm
- Appropriateness of care: That people receive appropriate, evidence-based care
- Partnering with consumers: That there are effective partnerships between consumers and healthcare providers
https://www.safetyandquality.gov.au/national-priorities/goals/

